- QMS validation ensures regulatory compliance and software reliability, crucial for regulated industries like pharmaceuticals and medical devices, but valuable for lesser/non-regulated industries as well.
- Effective strategies include leveraging vendor resources, optimizing documentation, and maintaining ongoing validation processes.
- Risk assessments and streamlined validation protocols improve compliance, audit readiness, and quality assurance.
In regulated industries such as pharmaceuticals, medical devices, and food manufacturing, validation of Quality Management Systems (QMS) ensures compliance with standards like FDA 21 CFR Part 11, ISO 13485, and EU MDR. (These tools, while not required in less regulated industries, are valuable for maintaining product/service integrity and can be used as models there.)
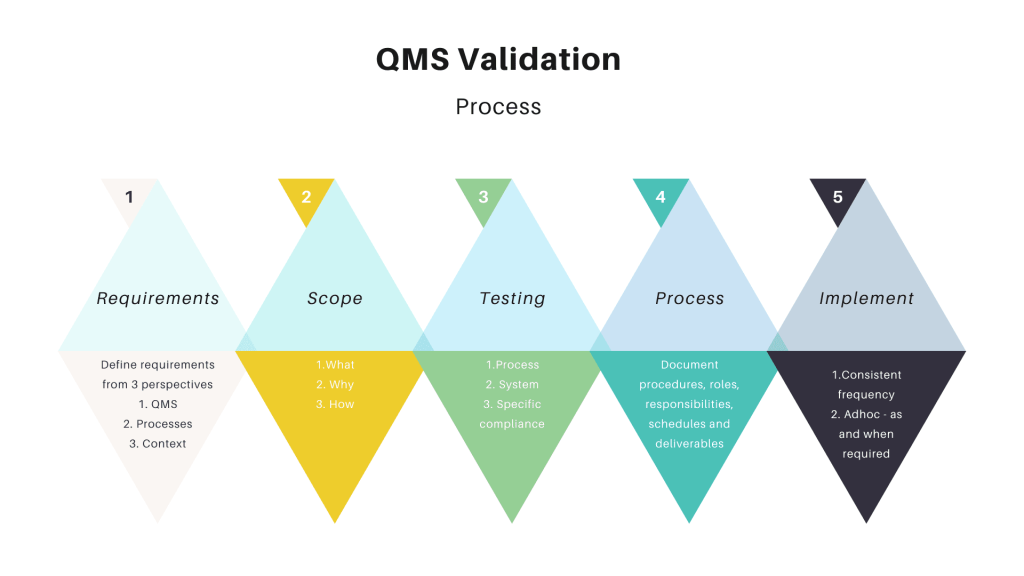
Validation confirms that QMS software functions correctly and aligns with regulatory requirements, safeguarding data integrity and ensuring product quality. Key strategies for effective validation include using vendor-provided pre-validated templates, optimizing documentation with concise records, and centralizing test cases for efficiency.
Validation steps involve creating a User Requirements Specification (URS), conducting risk assessments, and developing detailed validation plans and protocols. These processes ensure thorough testing of critical functionalities and adherence to regulatory standards. Tools such as traceability matrices link requirements to testing outcomes, strengthening validation reliability and audit preparedness. Continuous validation processes, including regular reviews and re-validation, maintain compliance and adapt to regulatory updates.


Leave a Reply
You must be logged in to post a comment.